Plain Language Summary
Mercury is all around us in the environment and we all have small amounts of mercury in our bodies. There are different forms of mercury, and each form is absorbed into the body differently. All forms of mercury and its metabolites are toxic, but mercury has been historically useful via its role in fluorescent lamps, thermometers, dental amalgams, vaccines, cosmetics, and explosives. Elemental mercury has been used as an ingredient in dental amalgams (the material used to fill cavities) for centuries, along with silver, copper, and tin. The problem is that the mercury used in dental fillings releases vapors, resulting in human exposure to this toxic substance. There have been many advances in the dental industry that minimize mercury exposure, including porcelain crowns, mercury-free filling materials, such as composite resins (“white fillings”), and using specialized equipment during extraction. These newer advancements should be used in place of mercury fillings when available. If mercury fillings are the only option, or if they are already in place, it is essential to understand that mercury toxicity is highly dose-dependent. Low levels of mercury are released over time, but mothers with new or old mercury fillings can safely breastfeed. Placing new mercury fillings will result in lower mercury exposure to a breastfed infant than if a mercury filling is removed, but the infant’s risk is still low.
During amalgam restoration, mercury vapor is released as it is drilled out, but the toxic vapors are mostly captured by specialized dental equipment to protect you and the dental team. Mom’s mercury levels will go up temporarily. Most of the tiny amounts of mercury in breastmilk would be eliminated in the baby’s feces, just as it is when mom eats high-mercury fish. Overall, breastfeeding after dental amalgam restoration is not likely to cause any issues when proper dental procedures are used. If you want to know more, we’ve broken down a step-by-step explanation of how risky mercury dental amalgams (and their removal) are during lactation.
Background: What is Mercury?
Mercury (Hg) is a chemical element that naturally occurs in the environment and is commonly found in household products. While most people are exposed to small levels of mercury (0.0003 mg/m3) in the environment, industrial workers and populations with a diet high in fish may experience greater levels of mercury exposure.1,2
Mercury exists in different forms, and some are safer than others. The table below summarizes the types of mercury and where they are commonly found.3
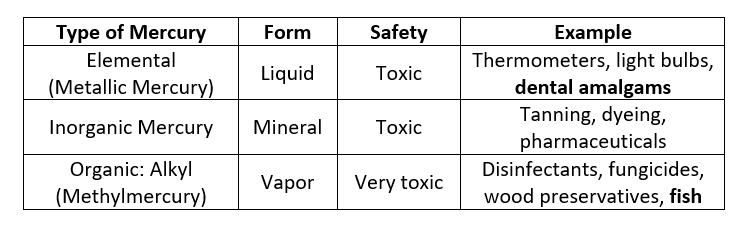
Mercury Poisoning
Mercury poisoning has been well recognized since the 17th century with the “Mad Hatter Syndrome” epidemic in European hat makers (CDC NIOSH). Even though all forms of mercury can be hazardous, some species tolerate mercury exposure better than others. For example, fish absorb methylmercury from water as they swim, and this mercury is tightly bound to their tissues. [Fun Fact: There is a study that shows high levels of mercury can affect the swim patterns of fish (Pereira 2016), but most literature focuses on mercury’s effects on humans rather than wildlife.]
Mercury toxicity in humans is dose-dependent. Chronic exposure to mercury may cause cardiovascular, reproductive and developmental toxicity, neurotoxicity, nephrotoxicity, immunotoxicity, and carcinogenicity.4 During acute exposure it is critical to assess for respiratory or cardiovascular complications. Similarly, people with abnormal kidney function or genetic polymorphisms related to mercury metabolism (GSTM1, GSTT1) may be less able to clear mercury from their bodies, putting them at higher risk of exceeding safe levels.5
Safe Levels of Mercury in the Human Body
Many federal organizations, such as the Occupational Safety and Health Administration (OSHA), the American Conference of Governmental Industrial Hygienists (ACGIH), and the Centers for Disease Control and Prevention (CDC), including the Agency for Toxic Substances and Disease Registry (ATSDR) and the National Institute for Occupational Safety and Health (NIOSH), have established guidelines regarding mercury exposure in humans.
Based on these recommendations and published case studies, the fatal mercury level in an adult is estimated to be 1429 mg/kg, and the acceptable blood mercury level has been defined as 0.02 mg/L (20 mcg/L).4 However, the United Nations Environment Programme (UNEP) and World Health Organization (WHO) established a more stringent limit of 0.005 mg/L (5 mcg/L) for mercury concentrations in both blood and urine in 2008.6
To put this in perspective, one serving (4 oz.) of salmon contains ~2.5 mcg of mercury (0.022 mcg of mercury per gram of fish). This means the average person would have to eat 40 servings of salmon (assuming 100% absorption and 0% metabolism/elimination of the mercury) to reach the blood concentration limit of 20 mcg/L. Similarly, one serving of tilapia contains ~1.5 mcg of mercury (0.013 mcg/g), so a person must eat more than 60 servings of tilapia to reach the defined limit.7
Based on a global study of mercury levels in humans from 1966 to 2015, the mercury levels in whole blood ranged from 0.75 mcg/L in Europe to 7.04 mcg/L in South America, with a worldwide blood mercury level averaging 1.58 mcg/L.8 These people likely were receiving mercury exposure from food, environment, and, potentially, dental amalgams. Even the highest average mercury concentration found,7.04 mcg/L, was significantly lower than the established acceptable blood mercury level of 20 mcg/L.
Mercury in Dental Fillings
Mercury’s chemical properties present an opportunity for dentists to mix a pliable amalgam to fit snugly in dental caries (cavities) that will harden into a solid, durable filling that delays tooth decay. Despite using elemental mercury in amalgam dental fillings since 1845, it has a toxic reputation.5 Its use in the general population, and breastfeeding moms in particular, has created questions about the safety of dental amalgam.
Dental amalgams are designed using elemental mercury, but they release mercury vapor over time. The odorless and invisible mercury vapor is significantly more dangerous than elemental mercury particles due to its rapid absorption and conversion to inorganic mercury, which may be trapped in the human brain and kidneys.1 The mercury vapor released from dental amalgams in humans results in constant exposure to this toxic substance. In 1991, the WHO stated that human exposure to mercury most commonly resulted from dental amalgams, and in 2017, it recognized mercury as one of the top ten chemicals of major concern for public health.5,9 Today, mercury exposure most frequently occurs due to consumption of organic methylmercury via fish and shellfish.2 However, many people still have dental amalgam fillings, and their contribution to mercury levels in humans should still be addressed.
Mercury Levels related to Dental Amalgams
Elemental mercury constitutes approximately half of a dental amalgam by weight.10 Each dental amalgam releases 2-5 mcg of mercury vapor per day in an average adult.11 However, despite concern regarding mercury exposure from tooth fillings, studies have shown that blood mercury levels due to exposure from dental amalgams are within the recommended limits.11
In a global study from 1966-2015 identified the main sources contributing to mercury exposure in humans to be environment, diet, dental amalgams, and occupation. This study identified that individuals with dental amalgams as having the lowest average blood mercury levels (0.48 mcg/L).8
Additionally, an Australian study determined that the average adult has eight teeth containing dental amalgams, resulting in approximately 28 mcg of mercury released from dental amalgams per day.11 The same study estimated that the average adult takes in 15 mcg of dietary mercury per day. Using these values, the average adult (with a 5-liter blood volume) would have an estimated (worst-case-scenario) blood mercury level of 8.6 mcg/L, which is still within the 20 mcg/L established exposure limit.
Mercury Exposure during Amalgam Replacement
Mercury exposure is significantly higher during dental amalgam restoration compared to initial placement.6 Is it enough to cause concern? Not really. The restoration process involves removing the previous filling and placing a new one, and therefore, mercury levels correlate with the number of dental amalgams being replaced.

This data suggests rapid absorption of mercury into the bloodstream and across the blood-brain barrier. [6]
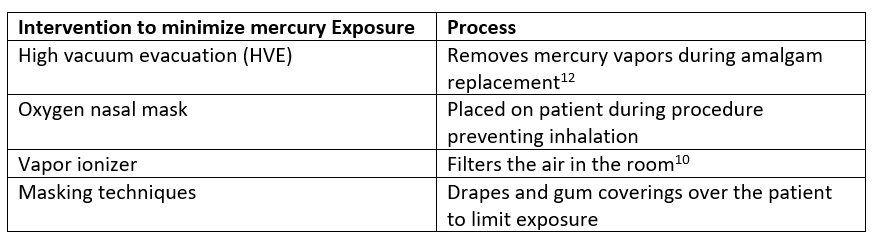
Each amalgam contains between 140-725 mg elemental mercury,13 however, in a restoration procedure using HVE, the primary focus is limiting exposure to mercury vapors because even if oral ingestion does occur, elemental mercury is typically not absorbed from the gastrointestinal tract. There is low risk of acute toxicity upon oral exposure, unless the patient has a GI fistula or inflammatory condition.4
Mercury vapor released from amalgams increases with the use of the high-speed drill in a dental procedure. During amalgam restoration, 1.4 mcg of mercury evaporates from freshly grated amalgam particulate per hour.14 In a 30-minute procedure, the average person could inhale 0.7 mcg of mercury vapor, which would contribute only 0.14 mcg/L to the blood volume of mercury. This leaves 0.35 mcg/L of mercury from other sources before reaching even the strictest acceptable level.
And Finally, Mercury in Lactation
Data suggests that maternal exposure to mercury vapor from dental amalgams or restoration is unlikely to have a significant impact on breastfeeding infants.
The established baseline for mercury levels in human breastmilk is set at ≥ 1 mcg/L.15 A comprehensive study examining mercury content in breast milk from mothers with dental amalgams revealed a range of mercury concentrations in human milk samples, spanning from < 0.2 to 6.86 mcg/L, with an average of 0.37 mcg/L.16 Studies have demonstrated transfer of mercury from a mother's bloodstream to her breastmilk. Intriguingly, mercury levels in commercial formula samples displayed a broader spectrum, varying from 0.4 to 2.5 mcg/L on average, which was actually higher than the findings in the collected breastmilk samples.16
Remember, even if materials from mercury amalgams are orally ingested, elemental mercury is not absorbed from a healthy gastrointestinal tract. If a baby consumes miniscule mercury residues in breastmilk, it is likely that it wouldn’t be absorbed, especially as the baby ages and the GI tract matures.
Conclusion
Breastfeeding with mercury fillings or after dental amalgam restoration is likely safe and the benefits of breastfeeding outweigh the risks. Extra-cautious mothers could choose not to breastfeed for the first three days post-amalgam restoration, but the difference in infant risk will be miniscule.
Nichole Campbell, MSN, APRN, NP-C
Kaytlin Krutsch, PharmD, BCPS
Edited from a previous draft written by Emily Conard, PharmD
References:
1. Mercury. Agency for Toxic Substances and Disease Registry. Updated October 14, 2015. Accessed December 11, 2023. https://www.atsdr.cdc.gov/sites/toxzine/mercury_toxzine.html
2. Mercury in dental amalgam. United States Environmental Protection Agency. Updated February 21, 2023. Accessed December 11, 2023. https://www.epa.gov/mercury/mercury-dental-amalgam
3. Mercury information. The Ohio State University. Accessed December 11, 2023. https://ehs.osu.edu/kb/mercury-information
4. Ye BJ, Kim BG, Jeon MJ, et al. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5. doi:10.1186/s40557-015-0086-8.
5. Jirau-Colón H, González-Parrilla L, Martinez-Jiménez J, Adam W, Jiménez-Velez B. Rethinking the dental amalgam dilemma: an integrated toxicological approach. Int J Environ Res Public Health. 2019;16(6). doi:10.3390/ijerph16061036.
6. Gul N, Khan S, Khan A, Nawab J, Shamshad I, Yu X. Quantification of Hg excretion and distribution in biological samples of mercury-dental-amalgam users and its correlation with biological variables. Environ Sci Pollut Res Int. 2016;23(20):20580-20590. doi:10.1007/s11356-016-7266-0.
7. Mercury levels in commercial fish and shellfish (1990-2012). United States Food and Drug Administration. Last reviewed February 25, 2022. Accessed December 11, 2023. https://www.fda.gov/food/environmental-contaminants-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
8. Sharma BM, Sáňka O, Kalina J, Scheringer M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ Int. 2019;125:300-319. doi:10.1016/j.envint.2018.12.016.
9. Santos-Sacramento L, Arrifano GP, Lopes-Araújo A, et al. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations. Ecotoxicol Environ Saf. 2021;208:111686. doi:10.1016/j.ecoenv.2020.111686.
10. Colson DG. A safe protocol for amalgam removal. J Environ Public Health. 2012;2012:517391. doi:10.1155/2012/517391.
11. Spencer AJ. Dental amalgam and mercury in dentistry. Aust Dent J. 2000;45(4):224-234. doi:10.1111/j.1834-7819.2000.tb00256.x.
12. Stone ME, Cohen ME, Berry DL, Ragain JC, Jr. Design and evaluation of a filter-based chairside amalgam separation system. Sci Total Environ. 2008;396(1):28-33. doi:10.1016/j.scitotenv.2008.02.037.
13. Adegbembo AO, Watson PA, Rokni S. Estimating the weight of dental amalgam restorations. J Can Dent Assoc. 2004;70(1):30.
14. Warwick D, Young M, Palmer J, Ermel RW. Mercury vapor volatilization from particulate generated from dental amalgam removal with a high-speed dental drill - a significant source of exposure. J Occup Med Toxicol. 2019;14:22. doi:10.1186/s12995-019-0240-2.
15. Al-Saleh I, Abduljabbar M, Al-Rouqi R, Elkhatib R, Alshabbaheen A, Shinwari N. Mercury (Hg) exposure in breast-fed infants and their mothers and the evidence of oxidative stress. Biol Trace Elem Res. 2013;153(1-3):145-154. doi:10.1007/s12011-013-9687-7.
16. Drasch G, Aigner S, Roider G, Staiger F, Lipowsky G. Mercury in human colostrum and early breast milk. Its dependence on dental amalgam and other factors. J Trace Elem Med Biol. 1998;12(1):23-27. doi:10.1016/s0946-672x(98)80017-5.